Pediatric inflammatory bowel disease (IBD) is no longer rare, and it’s no longer acceptable to treat it with outdated tools and timelines. Pediatric IBD accounts for approximately 25% of all newly diagnosed IBD patients. Clinical experience confirms that although the etiology of pediatric IBD and adult IBD are the same, in pediatrics, IBD often presents more aggressively than adult-onset disease. Consequently, we face a growing responsibility to ensure that children receive timely, effective, and personalized care. Yet with only two biologics (infliximab and adalimumab) currently approved for use down to age six, a rigid trial infrastructure, and limited investment in pediatric research, the system is falling short.
It’s time to change how we approach pediatric IBD, starting with smarter clinical trials, more flexible regulatory pathways, and tools like therapeutic drug monitoring (TDM) that help providers tailor treatment to each child’s needs.
The Growing Burden of Pediatric IBD
Pediatric IBD cases have risen significantly in the past decade. Very early-onset IBD (VEO-IBD), defined as diagnosis before age six, now accounts for 3–15% of pediatric cases. In some regions, the incidence of IBD in children under five is growing by as much as 7% per year. These trends reflect an urgent need to address pediatric IBD with the same urgency and innovation devoted to adult disease.
Approximately 25% of IBD cases now present in childhood or adolescence. These children face a greater cumulative disease burden and must manage a chronic condition for decades. VEO-IBD is often associated with unique genetic and immunologic underpinnings, complicating treatment further.
Did You Know?
The average time between adult and pediatric IBD drug approval is over seven years, despite the fact that 25% of all IBD cases begin in childhood. Kids can’t wait.
Some Specific Information About Pediatric IBD
While the etiology of IBD is similar across all age groups — a combination of genetic susceptibility, immune dysregulation, and environmental triggers — pediatric IBD often behaves differently. Crohn’s disease in children often presents with extensive GI involvement, rapid progression to complications, and a higher rate of surgery within the first five years of diagnosis. Pediatric ulcerative colitis similarly tends to involve the entire colon and may be more resistant to conventional therapies.
Children are also more susceptible to growth failure, delayed puberty, and psychosocial consequences from long-term disease. These factors make early, durable remission even more critical.
Limited Biologic Options for a Lifetime Disease
Children diagnosed with IBD at a young age will likely require biologic therapy well into adulthood. However, only two biologics — infliximab and adalimumab — are currently FDA-approved for pediatric IBD down to age six. Other newer therapies like vedolizumab, ustekinumab, and JAK inhibitors remain off-label for children, despite being mainstays in adult IBD care.
The average lag between adult and pediatric approval of biologics is seven years or more. To put this in context, the years of approval for MAb to treat IBD are shown below. Infliximab has the shortest time between approval for adults and pediatrics, eight years. Adalimumab took 12 years to be approved for pediatrics. Certolizumab was approved 17 years ago and still is not approved for pediatrics! The timelines for the other MAbs in this list are not much different.
Infliximab (Remicade)
Adults 1998 | Pediatric 2006
Adalimumab (Humira)
Adults 2002 | Pediatric 2014
Certolizumab pegol (Cimzia)
Adults 2008
Golimumab (Simponi)
Adults 2009
Ustekinumab (Stelara)
Adults 2009
Vedolizumab (Entyvio)
Adults 2014
Mirikizumab
Adults 2023
During this time, pediatric patients and providers must rely on off-label use and fight insurance denials to access therapies that are standard of care for adults. This delay can result in prolonged disease activity, increased risk of complications, and diminished quality of life.
The Reality of Pediatric Trial Design
Clinical and Ethical Challenges
Many pediatric patients eligible for clinical trials have already failed the two approved anti-TNF therapies. This makes it ethically untenable to randomize them to placebo arms or active comparators they’ve previously failed. Families and clinicians are understandably reluctant to enroll children in studies that offer no therapeutic benefit.
A 2024 systematic review of pediatric clinical trial networks showed a high rejection rate due to treatment preferences, such as using an active comparator instead of a placebo or a shorter study duration. However, even when active controls are used, they may not be clinically appropriate if the patient has already tried and failed both approved MAb therapies. The use of other MAbs as a control group isn’t feasible as they are not approved for use in pediatric patients.
Small, Fragmented Networks
Enrollment in pediatric trials is further limited by the fragmented nature of pediatric care. Most children are treated in community settings, not academic medical centers with clinical trial infrastructure.
For example, a 2022 FDA workshop found that out of a large multicenter pediatric IBD registry (~14,000 patients), only an estimated 5–10% had active moderate-to-severe disease that would meet typical trial inclusion criteria.
For Crohn’s disease trials, researchers projected only 749 children in that entire cohort would be eligible; for ulcerative colitis, only 488 children. Very early-onset patients (<6 years) were especially rare in these pools.
This means finding enough pediatric patients to adequately power a clinical trial is inherently difficult. Sponsors often must open many sites just to enroll a handful of patients at each center, driving up cost and complexity.
FDA Standards vs. Clinical Reality
The FDA’s preferred approach for pediatric studies — adequate and well-controlled trials with placebo or active comparators — is rarely feasible in this population. In practice, the pivotal pediatric studies for infliximab and adalimumab were single-arm trials that pooled pediatric safety and pharmacokinetic data with adult efficacy data.
Current FDA guidance acknowledges that placebo-controlled trials may pose risks in pediatric IBD, especially when effective therapies exist. Regulatory flexibility is essential to close the gap between adult and pediatric access to treatment.
The Case for Smarter Trial Design
To overcome these challenges, stakeholders must consider modern trial methodologies:
- Adaptive designs that initiate all patients on active therapy and randomize responders later.
- PK/PD modeling to extrapolate pediatric efficacy based on exposure-response relationships.
- Parallel trial timelines to begin pediatric studies during adult Phase III development.
- Master protocols and basket studies to evaluate multiple therapies efficiently.
These approaches not only reduce ethical concerns but also help pharma sponsors avoid costly, slow, and underpowered pediatric trials.

New Research: Baseline Clearance Predicts Outcomes
The VedoKids Study
A 2025 study published in Alimentary Pharmacology & Therapeutics analyzed 129 pediatric patients with IBD treated with vedolizumab. The study found that baseline drug clearance was a major determinant of treatment success, particularly deep biochemical remission at 30 weeks.
Key findings include:
- Patients with higher drug clearance had significantly lower remission rates. Only 31% of patients achieved deep biochemical remission, and high-clearance patients were more likely to fail to achieve biochemical remission.
- Lower albumin and lower weight were strong predictors of higher clearance. Prior biologic exposure also contributed to faster clearance.
- Standard weight-based dosing underperformed. Children ≤30kg received lower drug exposure than adults under typical regimens.
These findings demonstrate that children are not achieving adequate vedolizumab levels under current protocols and support more aggressive dosing strategies for high-clearance patients.
Why Weight-based Dosing Falls Short
Children differ from adults not just in size but in how they process medications. Faster drug clearance in pediatric patients leads to subtherapeutic trough levels, increasing the risk of loss of response and antidrug antibody (ADA) development. These insights apply to other biologics, including infliximab and adalimumab, where loss of response is often driven by underexposure rather than immunogenicity alone.
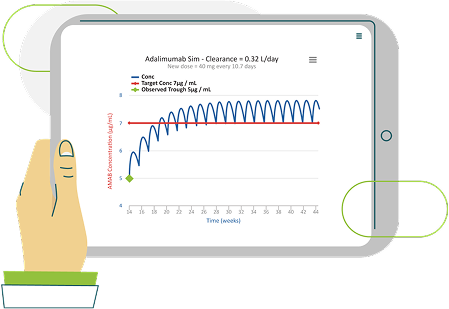
Precision Dosing Through Therapeutic Drug Monitoring (TDM)
The Value of Proactive TDM
Proactive TDM allows clinicians to maintain target drug levels and adjust therapy before loss of response occurs. By identifying patients at risk of suboptimal exposure early, clinicians can intensify therapy and reduce the likelihood of ADA formation and treatment failure.
This approach is especially critical in pediatrics, where faster clearance and disease aggressiveness demand closer monitoring and tighter control.
How iDose Enables Personalized Care
Baysient’s iDose platform uses Bayesian modeling to generate individualized dosing recommendations. By incorporating real-time data on weight, albumin, inflammatory markers, and clearance, iDose provides:
- Preemptive dose adjustments based on patient-specific risk factors.
- Improved durability of biologic therapy by minimizing underdosing and ADA development.
- Reduced healthcare costs through fewer flares, hospitalizations, and medication switches.
The VedoKids study reinforces the need for tools like iDose to translate pharmacokinetic insights into practical, clinic-ready solutions.
How You Can Help
Pediatric Gastroenterologists
- Use TDM and tools like iDose to optimize therapy from day one.
- Refer eligible patients to trials and advocate for smarter study designs.
- Educate families about the value of proactive dosing in long-term disease control.
FDA and Regulatory Agencies
- Embrace modeling, extrapolation, and adaptive designs for pediatric approvals.
- Recognize that placebo-controlled trials are not always ethical or feasible.
- Provide clear guidance on acceptable alternative pathways to approval.
Pharmaceutical Sponsors
- Design pediatric trials in parallel with adult development programs.
- Partner with pediatric networks to streamline enrollment.
- Invest in modeling and precision dosing to demonstrate pediatric value and safety.
Pediatric IBD Deserves More
The rise of pediatric and very early-onset IBD is a clear mandate for action. Children deserve timely access to new therapies, trial designs that reflect their reality, and dosing strategies that consider their unique biology.
With tools like Baysient’s iDose and a commitment from regulators, providers, and sponsors, we can change the standard of care for pediatric IBD. The science is here. The technology is here. The only thing left is the will to act.