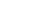IV Infliximab, a monoclonal antibody, has emerged as an efficient treatment with many clinical benefits, such as quick disease activity reduction and IBD patient life quality improvement. However, drug development of infliximab using weight-based dosing of MAbs without consideration of the variation in intrapatient clearance during treatment has resulted in an entire class of approved therapeutics suffering from the same problem: relatively high rates of primary nonresponse and secondary loss of response, often due to the development of anti-drug antibodies (ADA).
How It Works
This complex medication works by blocking the action of a protein in the body, called tumor necrosis factor-alpha (TNF-alpha), which is made by the body’s immune system. Individuals with certain conditions, such as inflammatory bowel disease (IBD) or rheumatoid arthritis (RA), have too much TNF-alpha. This causes the immune system to attack healthy parts of the body. With infliximab, the damage caused by too much TNF-alpha can be blocked.
Fixing Infliximab Dosing With Therapeutic Drug Monitoring
Modern medicine routinely follows standard dosing regimens that are established during drug development and are described in the product label. The labeled dosing is for the ‘typical patient’, whereas every patient is different and may need individualized dosing based on specific characteristics. The approved label on infliximab does not take into account the various factors that impact the way an individual’s body responds and processes these therapies.
Therapeutic drug monitoring (TDM) has been expressed in the use of dosing infliximab. TDM can estimate clearance values that are used to calculate individual patient dosing regimes for specific dosing levels.
Why are we monitoring clearance? Clearance and half-life are highly correlated, so a fast clearance indicates a short half-life. Patients with a short half-life will have non-measurable trough concentrations. These patients need a shorter dosing interval. For example, patients with acute severe ulcerative colitis can have a half-life as short as two days, which is why many require dose intensification.
Example:
Hypothetical Patient E has IBD, was biologic naïve, and started 5 mg/kg IFX treatment. E’s physician is using a treat-to-target approach in maintenance and has selected the following target trough:
- Maintenance trough concentrations: 3 to 10 µg/ml (i.e. from week 14 infusion and all infusions thereafter).
Figure I is patient E’s IFX concentration time profile for the first maintenance infusions.
Unknown information about patient E that might be useful if it were known:
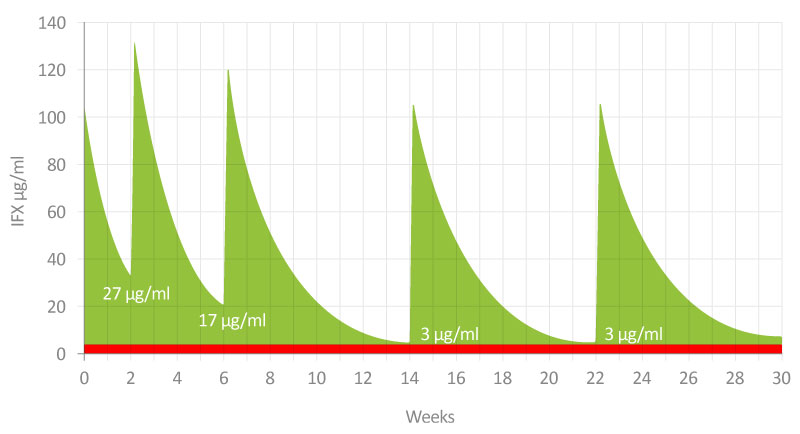
- The patient’s IFX half-life is 7.7 days, which is consistent with the half-life for IBD patients referenced in the prescribing information (package insert).
- Knowing that the drug will be largely cleared in 4-5 half-lives (roughly 39 days), a dose interval of 8 weeks will result in very low concentrations for about 2.5 weeks in each dose interval.
- During the 2.5 weeks that the patient has no circulating drug, the disease can progress and patients may also develop anti-drug antibodies.
Baysient’s New App: T3
The biological half-life value of the drug is defined as the time it takes for the concentration of the drug in the plasma or the total amount in the body to be reduced by 50%. Identifying the biological half-life value is useful for determining excretion rates as well as steady-state concentrations for the drug.
Baysient’s new app, T3 is a nomogram that will determine the biological half-life of a drug for each patient. By having this data, clinicians will have a precise estimate of their patient’s half-life, which will also provide an accurate indication of the length of time until the drug concentrations fall below the target concentration.
This web-based application was specifically developed to calculate precision dosing based on real patient’s clinical characteristics. Every patient is different, therefore, every patient may need individualized dosing.
Every clinician should be utilizing this nomogram when prescribing patients infliximab label drugs because it will help allow the drug to be present long enough and at a high enough exposure to treat their condition. In some cases, some medical conditions can either shorten or prolong the half-life of a drug, which is why it is important for clinicians to consider half-life when prescribing drugs to avoid over or under-dosing.
Infliximab Label
Despite infliximab’s therapeutic effects, roughly 10-30% of patients do not respond to the initial treatment and 23-46% of patients lose response over time. This occurs because the infliximab label doses to a “typical patient” and doesn’t take into account variability in patient clearance and half-life. According to research, “the standard infliximab maintenance dosing is 5 mg/kg every 8 weeks and has shown to be inadequate to consistently achieve sufficient drug exposure to minimize loss of response.”
Additionally to infliximab’s inadequate dosing regimen for maintenance, the infliximab label does not provide sufficient information on dose interval for patients with a very short half-life. In many cases, patients with a short half-life are encouraged to take more of the drug. However, increasing the dose doesn’t always fix the problem with patients with a short half-life and low troughs.
Optimized dosing strategies for individual patients are needed to achieve sufficient drug exposure during infliximab therapy. By focusing on important, modifiable factors for regulating drug levels, T3 can improve the efficacy of inflammatory disease treatments in clinical settings.

Why Choose T3?
The T3 system provides multiple dosing regimens that can allow patients in maintenance to attain and sustain a therapeutic drug trough level, improving their long-term drug response. T3 works by calculating treatment plans that yield a significantly lower incidence of anti-drug antibodies and treatment failures to help patients maintain appropriate coverage for drug maintenance, reach remission faster and remain asymptomatic.
This easy-to-use, intuitive app provides individualized maintenance dosing calculations at your fingertips.
Interested in how T3 can improve your patient outcomes? Get Started | Only $25 Per Month!